ISFM Guidelines
ISFM has developed practice guidelines designed to facilitate high standards of feline health care. These have been produced in collaboration with the American Association of Feline Practitioners (AAFP).
All the Guidelines are free to view and download and can be accessed via the JFMS website.
Blood pressure recommendations
 With the support of Ceva, ISFM has produced Practical recommendations on the measurement of indirect blood pressure in cats.
With the support of Ceva, ISFM has produced Practical recommendations on the measurement of indirect blood pressure in cats.
The recommendations cover:
- Procedures: Background, methods, patient and environment considerations
- Blood pressure assessment with the Doppler technique
- Blood pressure assessment with the HDO technique
- Blood pressure assessment forms
Blood and blood product collection and transfusion
ISFM has developed practice guidelines and resources for cat caregivers on this topic available here.
These resources cover:
- Blood groups and blood typing
- Collection of blood
- Administering blood or blood products
- Monitoring for and managing adverse reactions
- Suitability of cats as donors to ensure the best welfare outcomes for both patients
- Advice for cat caregivers
Downloadable Resources
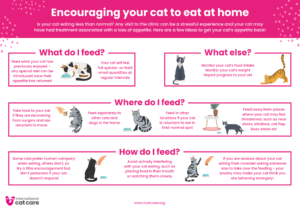
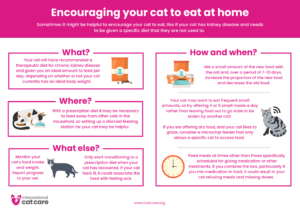
Encouraging your cat to eat at home – Download as an A4 Poster
Encouraging your cat to eat at home CKD version – Download as an A4 Poster
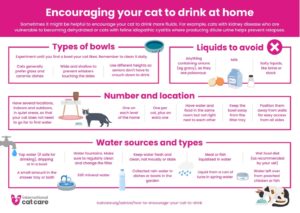
Encouraging your cat to drink at home – Download as an A4 Poster

Five pillars for making a healthy feline environment – Download the square social media graphic
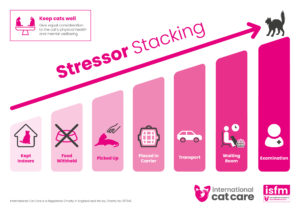
Stressor stacking – Download the infographic
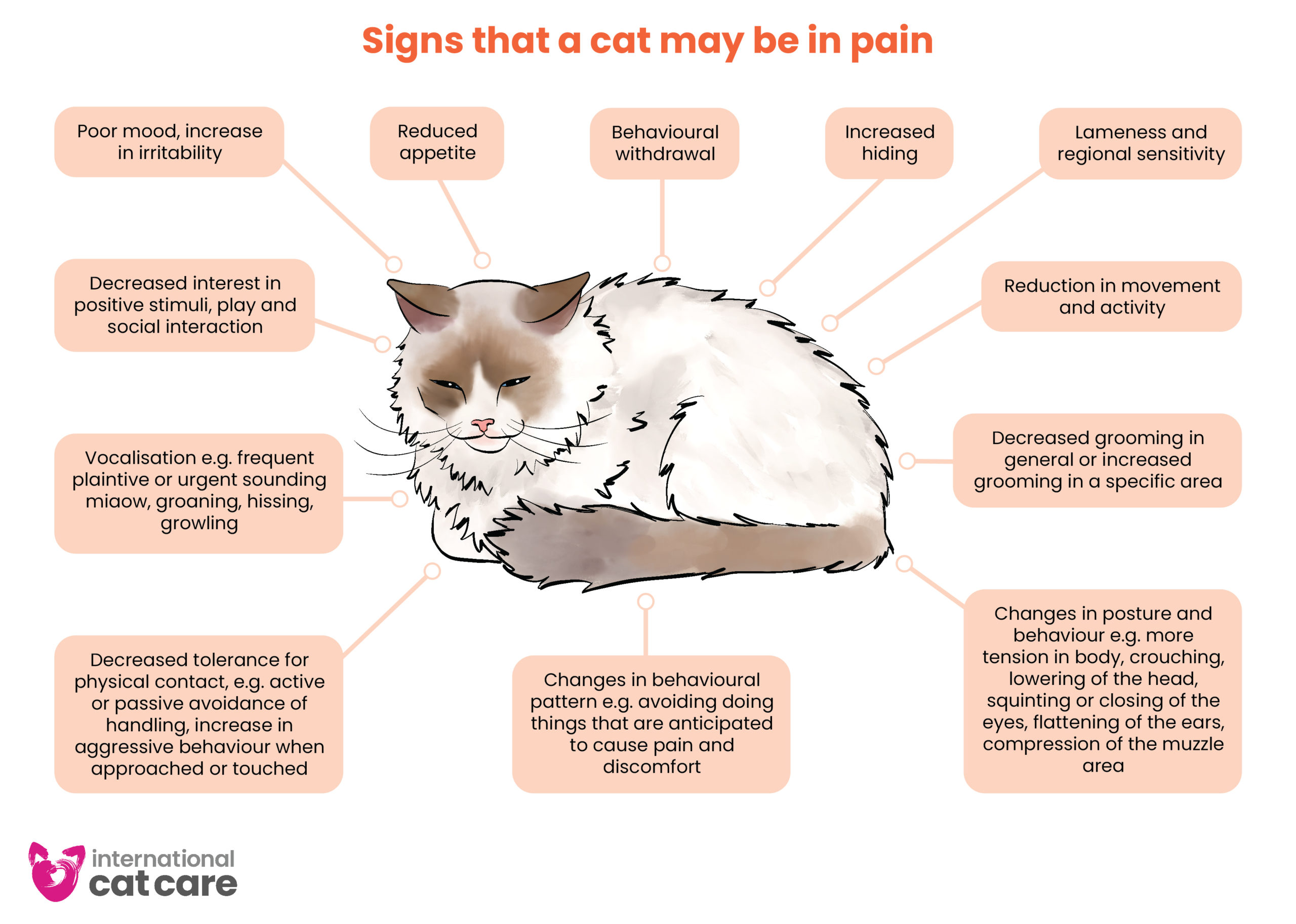
Signs of pain – Download the infographic
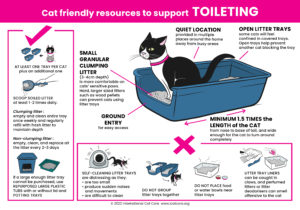
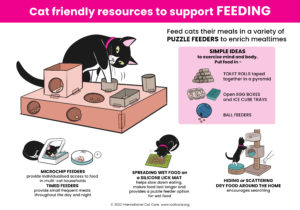
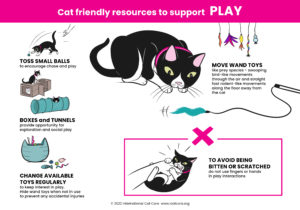
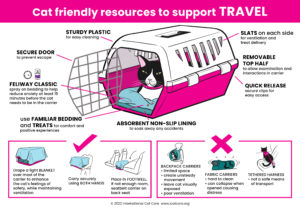
Cat Friendly Resources – Click the images to download the posters.
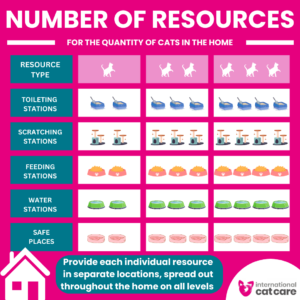
Number of resources – Download the infographic
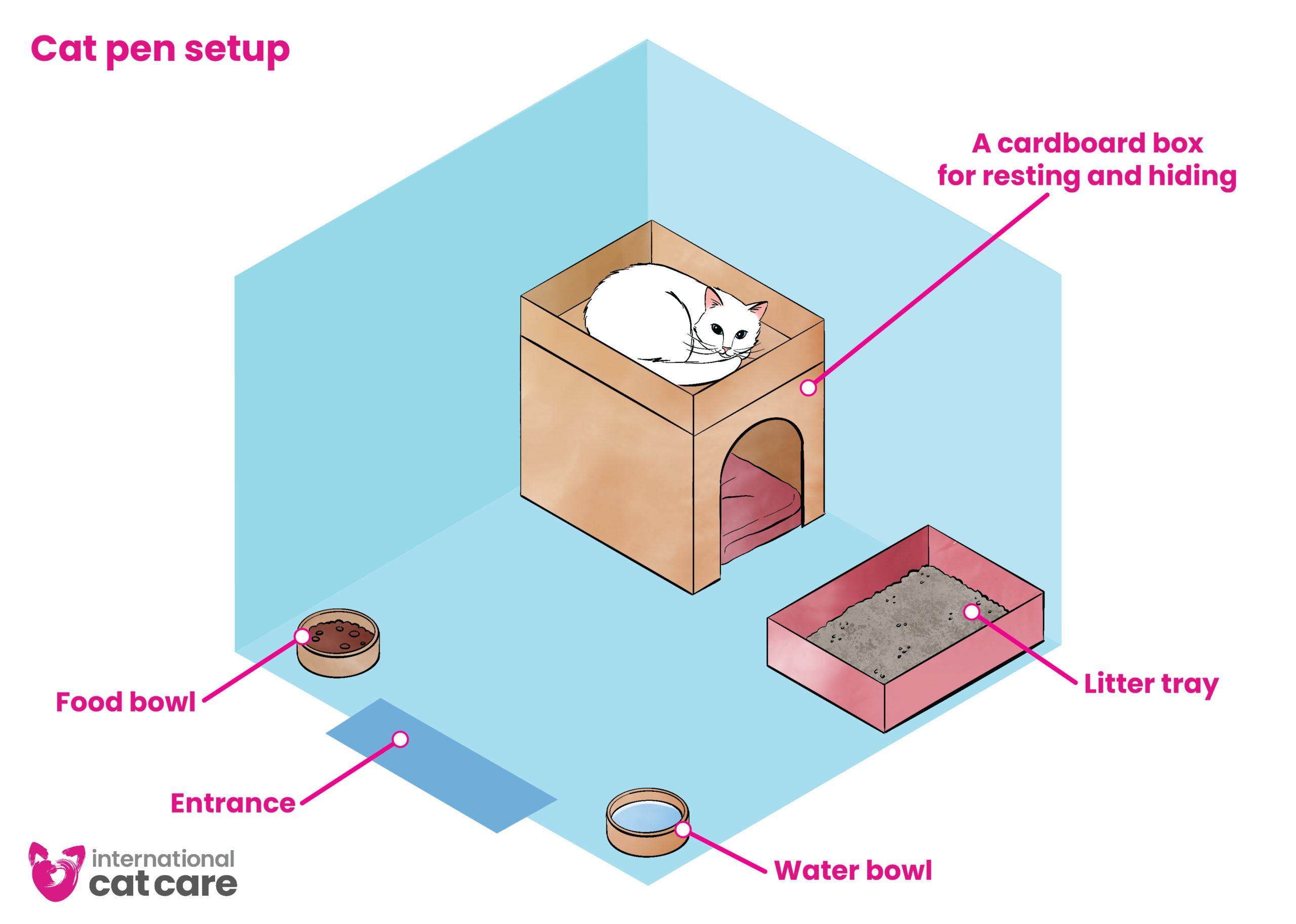
Cat pen setup – Download the infographic
Cat Friendly Handling Videos
 International Cat Care, in partnership with Ceva, has developed a set of handling videos for both vets and nurses, and for owners. The videos detail best practice handling techniques and approaches, and are designed to reduce stress for cats and improve safety in vet clinics, and also to help build a bond between owners and cats at home.
International Cat Care, in partnership with Ceva, has developed a set of handling videos for both vets and nurses, and for owners. The videos detail best practice handling techniques and approaches, and are designed to reduce stress for cats and improve safety in vet clinics, and also to help build a bond between owners and cats at home.
All our handling videos can be accessed on our YouTube channel
For owners
For owners, there are 13 videos covering two different areas, interacting with cats and handling cats. Particular attention is paid to getting cats used to going in the cat carrier and travelling – cats can be helped to understand that entering and spending time in their cat carrier is a positive experience. A number of the videos cover helping owners to get their cats accustomed to having ears, eyes, mouth, paws/claws and coat checked to improve handing at home, but also in the veterinary clinic. Of course, these are not just for owners, as the information is useful for the veterinary team to know in order to help owners prepare for veterinary visits as well as care at home.
Interacting with cats
- Approaching a cat
- How to touch and stroke a cat
- Things to avoid when handling a cat
- Handling your cat for grooming
- Handling kittens
Handling your cat at home
- Encouraging your cat to be happy in a cat carrier
- Getting your cat used to travel
- Putting your cat in a cat carrier
- Helping your cat to accept having its ears checked
- Helping your cat to accept having its mouth checked
- Helping your cat to accept having its coat checked
- Helping your cat to accept having its paws checked and claws clipped
- Helping your cat to accept having its eyes checked
For the veterinary clinic
Handling in the veterinary clinic
For the veterinary team, there are 16 videos covering many aspects of handling cats in the clinic, from assessing the cat while in the carrier to handling for blood sampling, intravenous catheter placement and administering oral, aural and ocular products. Tips on towel wrapping to assist handling in a comfortable way for the cat are also covered.
- Greeting and assessing the cat while it is in the carrier
- Removing a cat from the carrier
- Recognising and responding to signs of a happy cat
- Placing a cat in a cat carrier
- Performing a feline health examination
- Removing a cat from a veterinary cage
- Weighing a cat
- Towel wrapping a cat
- Taking a cat’s temperature
- Administering oral products to cats
- Administering cat spot-on products
- Administering aural products to cats
- Blood sampling a cat
- Intravenous catheter placement in a cat
- Cystocentesis
- Administering ocular products to cats